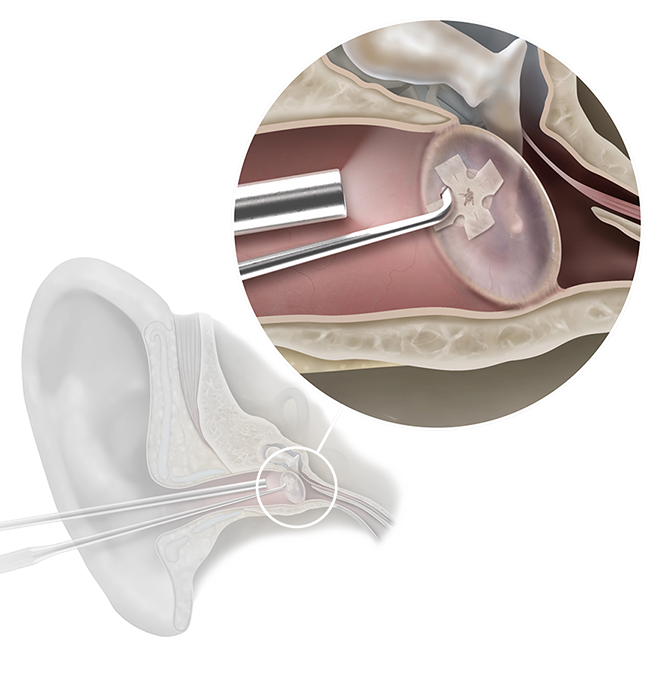
Biodesign® Otologic Butterfly Graft
A Transformative Approach to Traditional Butterfly Graft Tympanic Membrane Repair¹,²
The Biodesign Otologic Butterfly Graft is a modern, off-the-shelf option for tympanic membrane repair. Biodesign material supports a minimally invasive alternative to traditional butterfly graft techniques, eliminating the need for autologous tissue harvesting.³
Risk Information:
INTENDED USE: The Biodesign Otologic Butterfly Graft is intended for use as an implant material to aid in the natural healing process in myringoplasty and tympanoplasty procedures. The device is supplied sterile and is intended for one-time use.
CONTRAINDICATIONS: The device is derived from a porcine source and should not be used for patients with known sensitivity to porcine materials.
PRECAUTIONS: This device has only been studied in the indicated procedures using a trans canal approach in defects in the center of the tympanic membrane with no anatomical complications • Do not implant the device in the presence of an active infection • This device is designed for single use. Do not reprocess, resterilize, and/or reuse the device • Open the tray packaging carefully to ensure that the device remains seated in the tray • Discard the device if it has been implanted and removed. • After placement, pack ear canal with non-adherent dressings avoiding excessive pressure.
POTENTIAL COMPLICATIONS: Complications that can occur with the use of surgical device materials in otologic procedures may include, but are not limited to: • abscess formation • allergic reaction • calcification • cholesteatoma • discharge • excessive redness, pain, swelling, or blistering • fever • infection • inflammation • mastoiditis • migration • persistence of perforation • recurrence • reduced hearing • retraction pockets • seroma • squamous cysts • thickening of the tympanic membrane
VULNERABLE POPULATIONS: Safety data for this device has been collected in otherwise healthy populations. While no specific risks have been identified in vulnerable groups (e.g., patients with complex comorbidities or pregnancy), data in these populations is limited. Use in such cases should be guided by clinical judgment, including consultation with relevant specialists when appropriate.
Video Case Study:
Biodesign® Otologic Butterfly Repair Graft Ordering Information
| Order Number | Reference Part # | Size cm | Nominal Thickness mm |
| G60285 | ENT-OTO-BFLY-0.4-0.6 | 0.4,0.6 | 0.25 |
Biodesign® Otologic Butterfly Repair Graft IFU
References
1)Internal white paper; “Evaluation of industry opinion on
endoscopic transcanal TM repair as a conservative and
minimally invasive procedureˮ. Available on request.
2) https://www.accessdata.fda.gov/cdrh_docs/pdf23/K232646.pdf; Published K232646 510k summary describing device usability testing data in benchtop and animal models following an endoscopic transcanal approach.
3) D’Eredita R. Porcine small intestinal submucosa (SIS) myringoplasty in children: a randomized controlled study. Int J Pediatr Otorhinolaryngol. 2015;79(7):1085-1089.
® indicates U.S. trademark registration. All trademarks and/or images are the property of their respective owners or holders. Manufactured by Cook Biotech Incorporated.