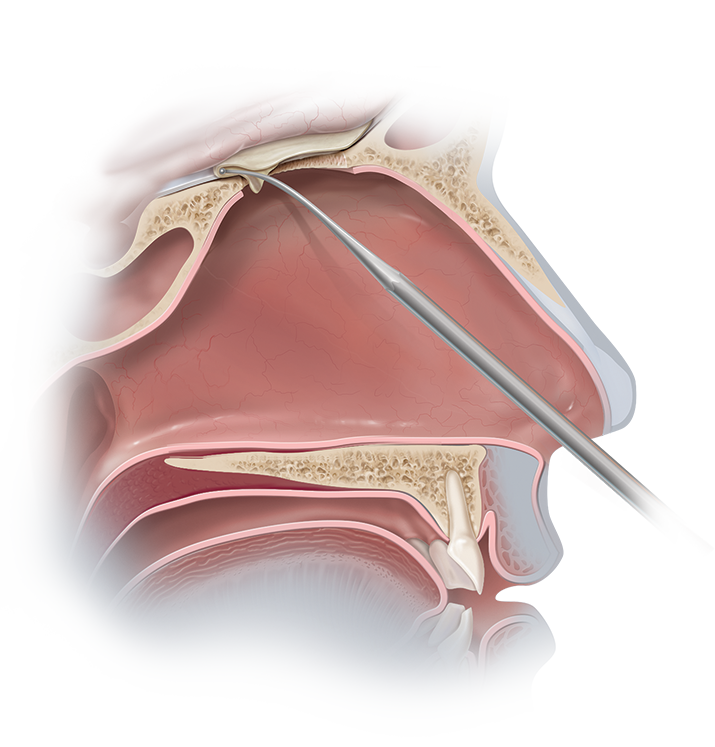
Biodesign® Duraplasty Graft remodels into host tissue to create a watertight seal.1,2
Biodesign Duraplasty Graft is used to repair the dura mater following surgical procedures (e.g. tumor resection). In other cases, the graft may be used to treat existing CSF leaks such as those resulting from trauma or sinus surgery.2,3
Biodesign material is easy to manipulate, doesn’t swell with hydration, and doesn’t adhere to itself when folded. It can be secured in place with or without sutures, depending on clinician preference.3
Biodesign® Duraplasty Graft Ordering Information
| Order Number | Reference Part # |
| G34977 | ENT-CBD-1X2 |
| G34978 | ENT-CBD-2.5X2.5 |
| G34979 | ENT-CBD-5X5 |
| G34980 | ENT-CBD-7X8.5 |
Biodesign® Duraplasty Graft IFU
Risk Information:
INTENDED USE: The Biodesign Duraplasty Graft is intended for use as a dura substitute for the repair of dura mater. This graft is supplied sterile in peel-open packages and intended for one-time use.
CONTRAINDICATIONS:
• Do not use in patients with a known sensitivity to porcine materials.
• Not for repair of spinal neural tube defects.
• Not for anterior spinal surgery with dural resection.
• Use with caution in infected regions.
• Covering defects involving mastoid cells is not recommended
• Not recommended for repair of large defects at the skull base following surgery; however, the graft may be used to augment other forms of specific repair.
PRECAUTIONS: In skull base repair procedures, the graft should not replace standard layering techniques or be implanted as a stand-alone repair • The graft is for single use only. Do not reprocess, resterilize, and/or reuse• If the graft is to be sutured, tensionless suturing technique is recommended • The trimmed graft should ensure an overlap to cover the existing dura• Ensure that all layers of the graft are secured if suturing the graft into place.
POTENTIAL COMPLICATIONS: Complications that can occur with the use of surgical graft materials in neurosurgical procedures may include, but are not limited to: • adhesion • allergic reaction • calcification • CSF leak • delayed hemorrhage • infection • inflammation
VULNERABLE POPULATIONS: Safety data for this device has been collected in otherwise healthy populations. While no specific risks have been identified in vulnerable groups (e.g., patients with complex comorbidities or pregnancy), data in these populations is limited. Use in such cases should be guided by clinical judgment, including consultation with relevant specialists when appropriate.
References
1) Cobb MA, Badylak SF, Janas W, Boop FA. Histology after dural grafting with small intestinal submucosa. Surg Neurol 1996;46:389-394
2) Bejjani GK, Zabramski J; Durasis Study Group. Safety and efficacy of the porcine small intestinal submucosa dural substitute: results of a prospective multicenter study and literature review. J Neurosurg. 2007;106(6):1028-1033.
3) Illing E, Chaaban MR, Riley KO, Woodworth BA. Porcine small intestine submucosal graft for endoscopic skull base reconstruction. Int Forum Allergy Rhinol. 2013;3(11):928-932.
® indicates U.S. trademark registration. All trademarks and/or images are the property of their respective owners or holders. Manufactured by Cook Biotech Incorporated.